Controlled Nucleation in Lyophilization
- vincenzo esposito

- Nov 25, 2019
- 2 min read
Updated: Jun 7, 2020
Nucleation is a dynamic process by which atoms or molecules aggregate to form clusters.
The classical theory of nucleation is based on the assumption that during the initial stages of the transformation, a few molecules rearrange themselves into droplets or nuclei that have the characteristics of the new phase. If the radius of these nuclei exceeds a certain radius to overcome the free energy barrier, then the growth of the new phase proceeds spontaneously.
Heterogeneous nucleation occurs on the surface of the object in question. The nucleus forms on the surface of the vapor or liquid, along with any bubbles or particles that may be provided with the nucleation site. Nucleation starts on the surface and spreads outward from there. That is why you can have a layer of ice in a bucket left outside in the cold, but still have liquid water underneath.
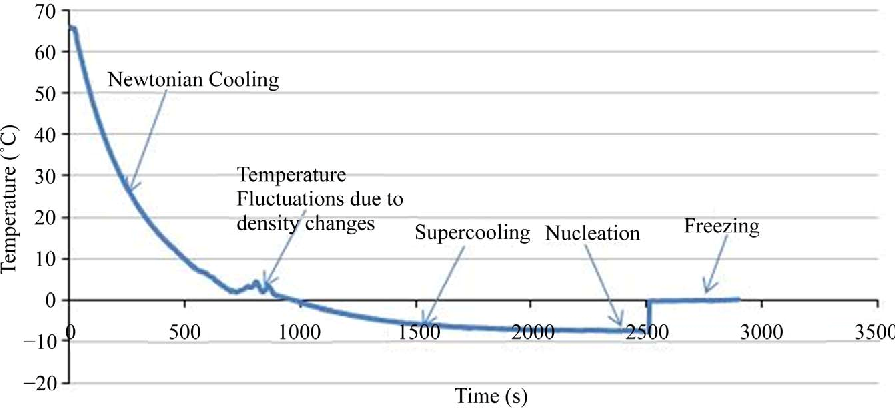
Homogenous nucleation occurs when there isn’t a preferential nucleation site. It is a more of a random occurrence, often spontaneous, because it does not have a preferential nucleation site. It is rare compared to heterogeneous nucleation because it requires the item in question to be either superheated or supercooled.
Controlled ice nucleation involves cooling the entire batch of vials to a given selected temperature that is below the equilibrium freezing point but above the temperature at which spontaneous heterogeneous nucleation may occur.
The effective nucleation temperature depends on several aspects. These factors include solution properties, process conditions, environmental influences, characteristics of the container and the presence of particulate matter.
An overview of the most relevant technical approaches follows:
The “ice fog” technique, where cold nitrogen gas is introduced into the chamber to form an “ice fog,” it involves purging cold nitrogen gas into the high humidity environment in the drying chamber to form an ice fog after the vials have achieved the temperature at which nucleation is desired.
Pressurizing the product chamber with an inert gas overpressure and a rapid release by evacuation. This method manipulates the chamber pressure of the freeze dryer to simultaneously induce nucleation in all product vials at a desired temperature.
Gap freezing approach, by inserting an air space between the vials and the shelf that eliminates significant conductive heat transfer from the shelf to the bottom of the vial.
Ultrasound-controlled ice nucleation. The concept uses a short acoustic pulse to induce crystal formation. This technology, however, never succeeded in a large scale environment.
Electro-freezing, to induce nucleation by using a strong electric pulse. This concept, however, also requires an electrode which is in direct contact with the product.
Controlling ice nucleation at a certain product temperature is expected to lead to a more uniform product because the degree of supercooling and nucleation temperature is influencing product parameters. Ice morphology decisively determines the resistance to water vapour flow from the ice sublimation interface through the already dried product during the drying process.






Comments